Five Major Developments in the Battle Against COVID
GoLocalProv News Team
Five Major Developments in the Battle Against COVID

While cases, transmission rate, and hospitalizations continue to run at high rates in Rhode Island, a number of developments have been announced that changes elements in the ongoing battle against the virus.
For the next few weeks, Rhode Island's numbers look to be deeply concerning.
GET THE LATEST BREAKING NEWS HERE -- SIGN UP FOR GOLOCAL FREE DAILY EBLASTOn Thursday, the RI Department of Health announced 1,379 cases, a transmission rate of 769.6, and 273 hospitalized -- all extremely high numbers.
The state also announced another three deaths pushing the total to 3,018.
Testing increased to 24,228 on Wednesday but was far short of demand.
1) RI Continues to Lead Country in New Cases Per Capita
Rhode Island continues to lead the country in the number of cases per 100,000. See the latest chart from Covid Act Now that shows how Rhode Island compares with the rest of New England.

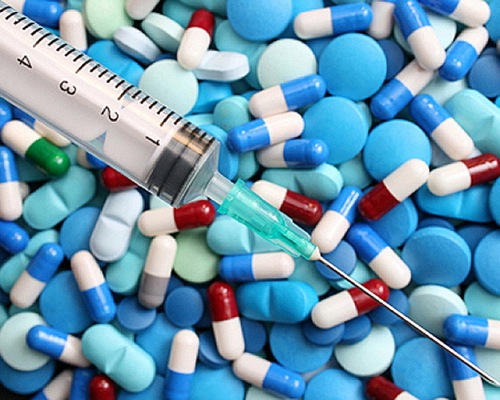
On Thursday, the FDA approved a Covid-19 pill from Merck & Co. and partner Ridgeback Biotherapeutics LP, the latest easy-to-use therapy that infected people can take to keep out of the hospital. On Wednesday, the agency had approved a similar treatment from Pfizer.
The authorization by the U.S. Food and Drug Administration permits doctors to prescribe the drug, called molnupiravir, to adults at high risk of severe disease shortly after they develop mild to moderate symptoms.
“The FDA recommended using the Merck drug only if other authorized drugs aren’t available and medically appropriate. Health experts have raised safety concerns about the Merck drug, which also was less effective in testing than an antiviral from Pfizer Inc,’ Reports the Wall Street Journal.
Merck’s drug was found to be 30% effective in reducing the risk of hospitalization and death in a key study, while Pfizer said Paxlovid was 89% effective at reducing risk of hospitalization and death in a key study. Both drugs were tested before the Omicron variant emerged.
{images_4}3) Policy Change by CDC on Isolation Period for Healthcare Workers
With rising concern over hospital staffing shortages as Omicron cases rapidly spread, the Centers for Disease Control and Prevention on Thursday shortened isolation periods for health care workers who contract COVID-19.
The agency recommended that health care workers who are asymptomatic return to work after seven days and a negative test, adding that “isolation time can be cut further if there are staffing shortages.”
Here is the CDC guidance issued Thursday regarding infected healthcare professionals (HCP) and isolation:
Due to concerns about increased transmissibility of the SARS-CoV-2 Omicron variant, this guidance is being updated to enhance protection for healthcare personnel (HCP), patients, and visitors, and to address concerns about potential impacts on the healthcare system given a surge of SARS-CoV-2 infections. These updates will be refined as additional information becomes available to inform recommended actions.
Ensure that SARS-CoV-2 testing is performed with a test that is capable of detectingexternal icon SARS-CoV-2, even with currently circulating variants in the United States.
Updated recommendations regarding when HCP with SARS-CoV-2 infection could return to work
The definition of higher-risk exposure was updated to include use of a facemask (instead of a respirator) by HCP if the infected patient is not also wearing a facemask or cloth mask.
Added options that would allow asymptomatic HCP with a higher-risk exposure who have not received all COVID-19 vaccine doses, including booster dose, as recommended by CDC to return to work prior to the previously recommended 14-day post-exposure period of work restriction, assuming they do not develop symptoms or test positive for SARS-CoV-2.
4} Omicron Less Likely to Need Hospitalization
The UK Health Security Agency says its early findings are "encouraging" but the variant could still lead to large numbers of people in hospital, according to the BBC.
"The study also shows the jab's ability to stop people catching Omicron starts to wane 10 weeks after a booster dose. Protection against severe disease is likely to be far more robust. The report comes hot on the heels of data from South Africa, Denmark, England and Scotland which all pointed to reduced severity," BBC reports.
5) Home Testing for All Americans Promised
The Biden administration announced this week that they have contracted for 500 million home test kits. The details are now being developed -- it is expected that a signup will be announced in early January and kits will begin to be mailed out during the month.

